Abstract
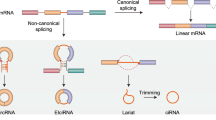
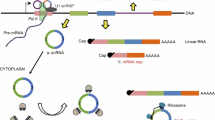
Circular RNAs (circRNAs) are covalently closed single-stranded RNA molecules derived from exons by alternative mRNA splicing. Circularization of single-stranded RNA molecules was already described in 1976 for viroids in plants. Since then several additional types of circular RNAs in many species have been described such as the circular single-stranded RNA genome of the hepatitis delta virus (HDV) or circular RNAs as products or intermediates of tRNA and rRNA maturation in archaea. CircRNAs are generally formed by covalent binding of the 5′ site of an upstream exon with the 3′ of the same or a downstream exon. Meanwhile, two different models of circRNA biogenesis have been described, the lariat or exon skipping model and the direct backsplicing model. In the lariat model, canonical splicing occurs before backsplicing, whereas in the direct backsplicing model, the circRNA is generated first. In this chapter, we will review the formation of circular RNAs and highlight the derivation of different types of circular RNAs.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Hsu MT, Coca-Prados M (1979) Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature 280:339–340
Nigro JM, Cho KR, Fearon ER et al (1991) Scrambled exons. Cell 64:607–613
Zaphiropoulos PG (1997) Exon skipping and circular RNA formation in transcripts of the human cytochrome P-450 2C18 gene in epidermis and of the rat androgen binding protein gene in testis. Mol Cell Biol 17:2985–2993
Cocquerelle C, Mascrez B, Hétuin D et al (1993) Mis-splicing yields circular RNA molecules. FASEB J 7:155–160
Cocquerelle C, Daubersies P, Majérus MA et al (1992) Splicing with inverted order of exons occurs proximal to large introns. EMBO J 11:1095–1098
Burd CE, Jeck WR, Liu Y et al (2010) Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet 6:e1001233
Aebi M, Weissman C (1987) Precision and orderliness in splicing. Trends Genet 3:102–107
Zhang Y, Zhang XO, Chen T et al (2013) Circular intronic long noncoding RNAs. Mol Cell 51:792–806
Dubin RA, Kazmi MA, Ostrer H (1995) Inverted repeats are necessary for circularization of the mouse testis Sry transcript. Gene 167:245–248
Sanger HL, Klotz G, Riesner D et al (1976) Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A 73:3852–3856
Schultz ES, Folsom D (1923) A “spindling-tuber disease” of Irish potatoes. Science 57:149. https://doi.org/10.1126/science.57.1466.149
Diener TO (1971) Potato spindle tuber “virus”. IV. A replicating, low molecular weight RNA. Virology 45:411–428
Stoler MH, Broker TR (1986) In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum Pathol 17:1250–1258
Summers J, Jones SE, Anderson MJ (1983) Characterization of the genome of the agent of erythrocyte aplasia permits its classification as a human parvovirus. J Gen Virol 64(Pt 11):2527–2532
Kos A, Dijkema R, Arnberg AC et al (1986) The hepatitis delta (delta) virus possesses a circular RNA. Nature 323:558–560
Soma A, Onodera A, Sugahara J et al (2007) Permuted tRNA genes expressed via a circular RNA intermediate in Cyanidioschyzon merolae. Science 318:450–453
Lykke-Andersen J, Aagaard C, Semionenkov M et al (1997) Archaeal introns: splicing, intercellular mobility and evolution. Trends Biochem Sci 22:326–331
Salgia SR, Singh SK, Gurha P et al (2003) Two reactions of Haloferax volcanii RNA splicing enzymes: joining of exons and circularization of introns. RNA 9:319–330
Danan M, Schwartz S, Edelheit S et al (2012) Transcriptome-wide discovery of circular RNAs in Archaea. Nucleic Acids Res 40:3131–3142
Tang TH, Rozhdestvensky TS, d’Orval BC et al (2002) RNomics in Archaea reveals a further link between splicing of archaeal introns and rRNA processing. Nucleic Acids Res 30:921–930
Jeck WR, Sorrentino JA, Wang K et al (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19:141–157
Barrett SP, Wang PL, Salzman J (2015) Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife 4:e07540
Diener TO, Raymer WB (1967) Potato spindle tuber virus: a plant virus with properties of a free nucleic acid. Science 158:378–381
Flores R, Hernández C, Martínez de Alba AE et al (2005) Viroids and viroid-host interactions. Annu Rev Phytopathol 43:117–139
Bonfiglioli, McFadden, Symons RH (1994) In situ hybridization localizes avocado sunblotch viroid on chloroplast thylakoid membranes and coconut cadang cadang viroid in the nucleus. Plant J 6(1):99–103
Przybilski R, Gräf S, Lescoute A et al (2005) Functional hammerhead ribozymes naturally encoded in the genome of Arabidopsis thaliana. Plant Cell 17:1877–1885
Steger G, Perreault JP (2016) Structure and associated biological functions of viroids. Adv Virus Res 94:141–172
Flores R, Grubb D, Elleuch A et al (2011) Rolling-circle replication of viroids, viroid-like satellite RNAs and hepatitis delta virus: variations on a theme. RNA Biol 8(2):200–206
Flores R, Gas ME, Molina-Serrano D et al (2009) Viroid replication: rolling-circles, enzymes and ribozymes. Viruses 1:317–334
Branch AD, Robertson HD (1984) A replication cycle for viroids and other small infectious RNA’s. Science 223:450–455
Flores (1999) Viroids with hammerhead ribozymes: some unique structural and functional aspects with respect to other members of the group. Biol Chem 380(7-8):849–854
Macnaughton TB, Shi ST, Modahl LE et al (2002) Rolling circle replication of hepatitis delta virus RNA is carried out by two different cellular RNA polymerases. J Virol 76:3920–3927
Reid CE, Lazinski DW (2000) A host-specific function is required for ligation of a wide variety of ribozyme-processed RNAs. Proc Natl Acad Sci U S A 97:424–429
Petkovic S, Müller S (2015) RNA circularization strategies in vivo and in vitro. Nucleic Acids Res 43:2454–2465
Barral PM, Sarkar D, Su ZZ et al (2009) Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: key regulators of innate immunity. Pharmacol Ther 124:219–234
Yoneyama M, Onomoto K, Jogi M et al (2015) Viral RNA detection by RIG-I-like receptors. Curr Opin Immunol 32:48–53
Abelson J, Trotta CR, Li H (1998) tRNA splicing. J Biol Chem 273:12685–12688
Knapp G, Ogden RC, Peebles CL et al (1979) Splicing of yeast tRNA precursors: structure of the reaction intermediates. Cell 18:37–45
Lu Z, Filonov GS, Noto JJ, Schmidt CA, Hatkevich TL, Wen Y, Jaffrey SR, Matera AG (2015) Metazoan tRNA introns generate stable circular RNAs in vivo. RNA 21:1554–1565
Suzuki H, Zuo Y, Wang J et al (2006) Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res 34:e63
Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO (2012) Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One 7:e30733
Lasda E, Parker R, Parker ROY (2014) Circular RNAs : diversity of form and function. RNA 20:1829–1842
Grabowski PJ, Zaug AJ, Cech TR (1981) The intervening sequence of the ribosomal RNA precursor is converted to a circular RNA in isolated nuclei of Tetrahymena. Cell 23:467–476
Durovic P, Dennis PP (1994) Separate pathways for excision and processing of 16S and 23S rRNA from the primary rRNA operon transcript from the hyperthermophilic archaebacterium Sulfolobus acidocaldarius: similarities to eukaryotic rRNA processing. Mol Microbiol 13:229–242
Dennis PP, Ziesche S, Mylvaganam S (1998) Transcription analysis of two disparate rRNA operons in the halophilic archaeon Haloarcula marismortui. J Bacteriol 180:4804–4813
Kruger K, Grabowski PJ, Zaug AJ et al (1982) Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31:147–157
Grabowski PJ, Seiler SR, Sharp PA (1985) A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell 42:345–353
Qian L, Vu MN, Carter M, Wilkinson MF (1992) A spliced intron accumulates as a lariat in the nucleus of T cells. Nucleic Acids Res 20:5345–5350
Tabak HF, Van der Horst G, Smit J et al (1988) Discrimination between RNA circles, interlocked RNA circles and lariats using two-dimensional polyacrylamide gel electrophoresis. Nucleic Acids Res 16:6597–6605
Hedberg A, Johansen SD (2013) Nuclear group I introns in self-splicing and beyond. Mob DNA 4:1
Lambowitz AM, Zimmerly S (2011) Group II introns: mobile ribozymes that invade DNA. Cold Spring Harb Perspect Biol 3:1–19
Lehmann K, Schmidt U (2003) Group II introns: structure and catalytic versatility of large natural ribozymes. Crit Rev Biochem Mol Biol 38:249–303
Li-Pook-Than J, Bonen L (2006) Multiple physical forms of excised group II intron RNAs in wheat mitochondria. Nucleic Acids Res 34:2782–2790
Soesanto W, Lin HY, Hu E et al (2009) Mammalian target of rapamycin is a critical regulator of cardiac hypertrophy in spontaneously hypertensive rats. Hypertension 54:1321–1327
Capel B, Swain A, Nicolis S et al (1993) Circular transcripts of the testis-determining gene Sry in adult mouse testis. Cell 73:1019–1030
Pasman Z, Been MD, Garcia-Blanco MA (1996) Exon circularization in mammalian nuclear extracts. RNA 2:603–610
Caldas C, So CW, MacGregor A et al (1998) Exon scrambling of MLL transcripts occur commonly and mimic partial genomic duplication of the gene. Gene 208:167–176
Zaphiropoulos PG (1996) Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc Natl Acad Sci U S A 93:6536–6541
Baralle FE, Giudice J (2017) Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18:437–451
Salzman J, Chen RE, Olsen MN et al (2013) Cell-type specific features of circular RNA expression. PLoS Genet 9:e1003777
Zhang XO, Dong R, Zhang Y et al (2016) Diverse alternative back-splicing and alternative splicing landscape of circular RNAs. Genome Res 26:1277–1287
Zhang XO, Wang HB, Zhang Y et al (2014) Complementary sequence-mediated exon circularization. Cell 159:134–147
Chen LL (2016) The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 17:205–211
1000 Genomes Project Consortium, Auton A, Brooks LD et al (2015) A global reference for human genetic variation. Nature 526:68–74
Lynch M, Conery JS (2003) The origins of genome complexity. Science 302:1401–1404
Khanduja JS, Calvo IA, Joh RI, Hill IT, Motamedi M (2016) Nuclear noncoding RNAs and genome stability. Mol Cell 63:7–20
Böhmdorfer G, Wierzbicki AT (2015) Control of chromatin structure by long noncoding RNA. Trends Cell Biol 25:623–663
Magistri M, Faghihi MA, St Laurent G, Wahlestedt C (2012) Regulation of chromatin structure by long noncoding RNAs: focus on natural antisense transcripts. Trends Genet 28:389–396
Vance KW, Ponting CP (2014) Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet 30:348–355
Wilusz JE, Sunwoo H, Spector DL (2009) Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23:1494–1504
Bonasio R, Shiekhattar R (2014) Regulation of transcription by long noncoding RNAs. Annu Rev Genet 48:433–455
Lee TI, Young RA (2000) Transcription of eukaryotic protein-coding genes. Annu Rev Genet 34:77–137
Nogales E, Louder RK, He Y (2017) Structural insights into the eukaryotic transcription initiation machinery. Annu Rev Biophys 46:59–83
Jurado AR, Tan D, Jiao X et al (2014) Structure and function of pre-mRNA 5′-end capping quality control and 3′-end processing. Biochemistry 53:1882–1898
Moore MJ, Proudfoot NJ (2009) Pre-mRNA processing reaches back to transcription and ahead to translation. Cell 136:688–700
Furuichi Y, Shatkin AJ (2000) Viral and cellular mRNA capping: past and prospects. Adv Virus Res 55:135–184
Shandilya J, Roberts SGE (2012) The transcription cycle in eukaryotes: from productive initiation to RNA polymerase II recycling. Biochim Biophys Acta 1819:391–400
Wahle E, Rüegsegger U (1999) 3′-End processing of pre-mRNA in eukaryotes. FEMS Microbiol Rev 23:277–295
Frendewey D, Keller W (1985) Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell 42:355–367
Burset M, Seledtsov IA, Solovyev VV (2000) Analysis of canonical and non-canonical splice sites in mammalian genomes. Nucleic Acids Res 28:4364–4375
Hwang DY, Cohen JB (1996) Base pairing at the 5′ splice site with U1 small nuclear RNA promotes splicing of the upstream intron but may be dispensable for slicing of the downstream intron. Mol Cell Biol 16:3012–3022
Liu J, Liu T, Wang X, He A (2017) Circles reshaping the RNA world: from waste to treasure. Mol Cancer 16:58
Huang S, Yang B, Chen BJ et al (2017) The emerging role of circular RNAs in transcriptome regulation. Genomics 109:401–407
Starke S, Jost I, Rossbach O, Schneider T et al (2015) Exon circularization requires canonical splice signals. Cell Rep 10:103–111
Gilbert W (1978) Why genes in pieces? Nature 271:501
Valdivia HH (2007) One gene, many proteins: alternative splicing of the ryanodine receptor gene adds novel functions to an already complex channel protein. Circ Res 100:761–763
George CH, Rogers SA, Bertrand BMA et al (2007) Alternative splicing of ryanodine receptors modulates cardiomyocyte Ca2+ signaling and susceptibility to apoptosis. Circ Res 100:874–883
Lanner JT, Georgiou DK, Joshi AD, Hamilton SL (2010) Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2:a003996
Mabon SA, Misteli T (2005) Differential recruitment of pre-mRNA splicing factors to alternatively spliced transcripts in vivo. PLoS Biol 3:1893–1901
Boeckel J-N, Jaé N, Heumüller AW et al (2015) Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ Res 117(10):884–890
Konarska MM, Padgett RA, Sharp PA (1985) Trans splicing of mRNA precursors in vitro. Cell 42:165–171
Liang D, Wilusz JE (2014) Short intronic repeat sequences facilitate circular RNA production. Genes Dev 28:2233–2247
Lev-Maor G, Ram O, Kim E et al (2008) Intronic Alus influence alternative splicing. PLoS Genet 4:1–12
Hu S, Wang X, Shan G (2016) Insertion of an Alu element in a lncRNA leads to primate-specific modulation of alternative splicing. Nat Struct Mol Biol 23:1011–1019
Barrett SP, Salzman J (2016) Circular RNAs: analysis, expression and potential functions. Development 143:1838–1847
Braun S, Domdey H, Wiebauer K (1996) Inverse splicing of a discontinuous pre-mRNA intron generates a circular exon in a HeLa cell nuclear extract. Nucleic Acids Res 24:4152–4157
Chen LL, Yang L (2015) Regulation of circRNA biogenesis. RNA Biol 12:381–388
Schindewolf C, Braun S, Domdey H (1996) In vitro generation of a circular exon from a linear pre-mRNA transcript. Nucleic Acids Res 24:1260–1266
Hesselberth JR (2013) Lives that introns lead after splicing. Wiley Interdiscip Rev RNA 4:677–691
Acknowledgments
This work was supported by the German Cardiac Society (Deutsche Gesellschaft für Kardiologie (DGK)) to Jes-Niels Boeckel.
Competing Financial Interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Eger, N., Schoppe, L., Schuster, S., Laufs, U., Boeckel, JN. (2018). Circular RNA Splicing. In: Xiao, J. (eds) Circular RNAs. Advances in Experimental Medicine and Biology, vol 1087. Springer, Singapore. https://doi.org/10.1007/978-981-13-1426-1_4
Download citation
DOI: https://doi.org/10.1007/978-981-13-1426-1_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-1425-4
Online ISBN: 978-981-13-1426-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)