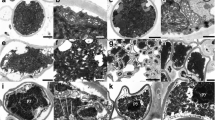
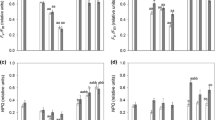
Abstract
Lichen desiccation/rehydration cycles lead to an increased oxidative stress modulated by the multifaceted mediator nitrogen monoxide (NO). Active cell death, frequently triggered by oxidative damage with NO participation, has been confirmed even in unicellular organisms. This adaptive mechanism has not been studied in lichens and no specific experimental protocols exist. Hoechst 33,342 enters viable cells and DNA binding increases its fluorescence, particularly intense in condensed apoptotic chromatin. YO-PRO-1 can only permeate the altered membrane of apoptotic P2X7-positive cells. Proteolytic caspases are activated upon different types of active cell death. Our objectives are to determine if these markers indicate active cell death in Ramalina farinacea after desiccation/rehydration and to study the effect of NO scavenging. YO-PRO-1, Hoechst 33342, and Caspase 3/7 Green DNA binding were assessed in thalli rehydrated with deionized water and with a cocktail of apoptosis inducers. A 24 h kinetics and a microscopical analysis were performed. YO-PRO-1 fluorescence was not detected, Hoechst 33342 staining abruptly decreases during the first hours, while caspase-like activity associated to phycobionts steadily increases. Whereas the apoptosis inducers cocktail 1x significantly increased caspase-like activity affecting both symbionts, Hoechst staining was only affected at 10x. NO scavenging diminishes caspase-like activation and seems to accelerate Hoechst abrupt decrease during thallus rehydration. In conclusion, the demonstration of caspase-like activity in R. farinacea and its Trebouxia phycobionts point to the presence of active cell death but other methods assessing cell effective death or DNA irreversible fragmentation (i.e. TUNEL assay) are necessary to confirm this feature.






Similar content being viewed by others
References
Beligni MV, Lamattina L (2001) Nitric oxide in plants: the history is just beginning: mini review. Plant Cell Environ 24:267–278. https://doi.org/10.1046/j.1365-3040.2001.00672.x
Beligni MV, Fath A, Bethke PC, Lamattina L, Jones RL (2002) Nitric oxide acts as an antioxidant and delays programmed cell death in barley Aleurone layers. Plant Physiol 129:1642–1650
Bidle KD (2016) Programmed cell death in unicellular phytoplankton. Curr Biol 26:R594–R607
Brüne B (2003) Nitric oxide: NO apoptosis or turning it ON? Cell Death Differ 10:864–869
Brüne B, Von Knethen A, Sandau KB (1998) Nitric oxide and its role in apoptosis. Eur J Pharmacol 351:261–272. https://doi.org/10.1016/S0014-2999(98)00274-X
Cai X (2012) P2X receptor homologs in basal fungi. Purinergic Signal 8:11–13
Cánovas D, Marcos JF, Marcos AT, Strauss J (2016) Nitric oxide in fungi: is there NO light at the end of the tunnel? Curr Genet 62:513–518. https://doi.org/10.1007/s00294-016-0574-6
Catalá M, Gasulla F, Pradas del Real AE et al (2010a) Nitric oxide is involved in oxidative stress during rehydration of Ramalina farinacea (L.) Ach. in the presence of the oxidative air pollutant cumene hydroperoxide. Bibl Lichenol 105:87–92
Catalá M, Gasulla F, Pradas del Real AE et al (2010b) Fungal-associated NO is involved in the regulation of oxidative stress during rehydration in lichen symbiosis. BMC Microbiol 10:297
Catalá M, Gasulla F, Pradas Del Real AE et al (2013) The organic air pollutant cumene hydroperoxide interferes with NO antioxidant role in rehydrating lichen. Environ Pollut 179:277–284
Collazo C, Chacón O, Borrás O (2006) Programmed cell death in plants resembles apoptosis of animals. Biotecnol Apl 23:1–10. https://doi.org/10.7150/ijbs.26962
Corpas FJ, Barroso JB (2015) Nitric oxide from a “green” perspective. Nitric Oxide 45:15–19
de Carpentier F, Lemaire SD, Danon A (2019) When unity is strength: the strategies used by chlamydomonas to survive environmental stresses. Cells 8:1307. https://doi.org/10.3390/cells8111307
de Pinto MC, Tommasi F, De Gara L (2002) Changes in the antioxidant systems as part of the signaling pathway responsible for the programmed cell death activated by nitric oxide and reactive oxygen species in tobacco bright-yellow 2 cells. Plant Physiol 130:698–708
Deponte M (2008) Programmed cell death in protists. Biochim Biophys Acta Mol Cell Res 1783:1396–1405. https://doi.org/10.1016/j.bbamcr.2008.01.018
Dickman M, Williams B, Li Y, de Figueiredo P, Wolpert T (2017) Reassessing apoptosis in plants. Nat Plants 3:773–779
Dunn SR, Thomason JC, Le Tissier MDA, Bythell JC (2004) Heat stress induces different forms of cell death in sea anemones and their endosymbiotic algae depending on temperature and duration. Cell Death Differ 11:1213–1222. https://doi.org/10.1038/sj.cdd.4401484
Expósito JR, Coello AJ, Barreno E et al (2020) Endogenous NO is involved in dissimilar responses to rehydration and Pb(NO3)2 in Ramalina farinacea Thalli and its isolated phycobionts. Microb Ecol 79:604–616. https://doi.org/10.1007/s00248-019-01427-2
Fujisawa S, Romin Y, Barlas A, Petrovic LM, Turkekul M, Fan N, Xu K, Garcia AR, Monette S, Klimstra DS, Erinjeri JP, Solomon SB, Manova-Todorova K, Sofocleous CT (2014) Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases. Cytotechnology 66:259–273
Ge Y, Cai YM, Bonneau L, Rotari V, Danon A, McKenzie EA, McLellan H, Mach L, Gallois P (2016) Inhibition of cathepsin B by caspase-3 inhibitors blocks programmed cell death in Arabidopsis. Cell Death Differ 23:1493–1501. https://doi.org/10.1038/cdd.2016.34
Gonçalves AP, Heller J, Daskalov A, Videira A, Glass NL (2017) Regulated forms of cell death in fungi. Front Microbiol:8. https://doi.org/10.3389/fmicb.2017.01837
Green DR, Victor B (2012) The pantheon of the fallen: why are there so many forms of cell death? Trends Cell Biol 22:555–556. https://doi.org/10.1016/j.tcb.2012.08.008
Hamann A, Brust D, Osiewacz HD (2008) Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol 16:276–283. https://doi.org/10.1016/j.tim.2008.03.003
Havel L, Durzan DJ (1996) Apoptosis in plants. Bot Acta 109:268–277
He H-Y, Gu M-H, He L-F (2014) The role of nitric oxide in programmed cell death in higher plants. In: Khan MN, Mobin M, Mohammad F, Corpas FJ (eds) Nitric oxide in plants: metabolism and role in stress physiology. Springer, pp 281–296
He H, Huang W, Oo TL, Gu M, Zhan J, Wang A, He LF (2018) Nitric oxide suppresses aluminum-induced programmed cell death in peanut (Arachis hypoganea L.) root tips by improving mitochondrial physiological properties. Nitric Oxide 74:47–55. https://doi.org/10.1016/j.niox.2018.01.003
He H, Oo TL, Huang W et al (2019) Nitric oxide acts as an antioxidant and inhibits programmed cell death induced by aluminum in the root tips of peanut (Arachis hypogaea L.). Sci Rep 9:1–12
Heath MC (1998) Apoptosis, programmed cell death and the hypersensitive response. Eur J Plant Pathol 104:117–124. https://doi.org/10.1023/A:1008645520976
Hengartner MO (2000) The biochemistry of apoptosis : abstract : nature. Nature 407:770–776
Kranner I, Birtic S (2005) A modulating role for antioxidants in desiccation tolerance. Integr Comp Biol 45:734–740
Kranner I, Birtić S, Anderson KM, Pritchard HW (2006) Glutathione half-cell reduction potential: a universal stress marker and modulator of programmed cell death? Free Radic Biol Med 40:2155–2165. https://doi.org/10.1016/j.freeradbiomed.2006.02.013
Leist M, Nicotera P (1997) The shape of cell death. Biochem Biophys Res Commun 236:1–9
Leslie JF, Zeller KA (1996) Heterokaryon incompatibility in fungi - more than just another way to die. J Genet 75:415–424. https://doi.org/10.1007/BF02966319
Lockshin RA, Zakeri Z (2004) Apoptosis, autophagy, and more. Int J Biochem Cell Biol 36:2405–2419
Martin SJ, Reutelingsperger CPM, McGahon AJ et al (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of BCL-2 and Abl. J Exp Med 182:1545–1556. https://doi.org/10.1084/jem.182.5.1545
Meilhoc E, Boscari A, Bruand C, Puppo A, Brouquisse R (2011) Nitric oxide in legume-Rhizobium symbiosis. Plant Sci 181:573–581. https://doi.org/10.1016/j.plantsci.2011.04.007
Mohammad K, Dakik P, Medkour Y, McAuley M, Mitrofanova D, Titorenko VI (2018) Yeast cells exposed to exogenous palmitoleic acid either adapt to stress and survive or commit to regulated liponecrosis and die. Oxid Med Cell Longev 2018. https://doi.org/10.1155/2018/3074769
Moharikar S, D’Souza JS, Kulkarni AB, Rao BJ (2006) Apoptotic-like cell death pathway is induced in unicellular chlorophyte Chlamydomonas reinhardtii (Chlorophyceae) cells following UV irradiation: detection and functional analyses. J Phycol 42:423–433. https://doi.org/10.1111/j.1529-8817.2006.00207.x
Murik O, Elboher A, Kaplan A (2014) Dehydroascorbate: a possible surveillance molecule of oxidative stress and programmed cell death in the green alga Chlamydomonas reinhardtii. New Phytol 202:471–484. https://doi.org/10.1111/nph.12649
Pandey SS, Singh S, Pathak C, Tiwari BS (2018) “Programmed cell death: a process of death for survival” – how far terminology pertinent for cell death in unicellular organisms. J Cell Death 11
Pepper JW, Shelton DE, Rashidi A, Durand PM (2013) Are internal, death-promoting mechanisms ever adaptive? J Phylogen Evol Biol 1
Raff M (1998) Cell suicide for beginners. Nature 396:119–122. https://doi.org/10.1038/24055
Rat P, Olivier E, Tanter C et al (2017) A fast and reproducible cell- and 96-well plate-based method for the evaluation of P2X7 receptor activation using YO-PRO-1 fluorescent dye. J Biol Methods 4:e64
Reape TJ, Molony EM, McCabe PF (2008) Programmed cell death in plants: distinguishing between different modes. J Exp Bot 59:435–444. https://doi.org/10.1093/jxb/erm258
Segovia M, Haramaty L, Berges JA, Falkowski PG (2003) Cell death in the unicellular chlorophyte Dunaliella tertiolecta. A hypothesis on the evolution of apoptosis in higher plants and metazoans. Plant Physiol 132:99–105. https://doi.org/10.1104/pp.102.017129.in
Serrano I, Romero-Puertas MC, Sandalio LM, Olmedilla A (2015) The role of reactive oxygen species and nitric oxide in programmed cell death associated with self-incompatibility. J Exp Bot 66:2869–2876
Sharon A, Finkelstein A, Shlezinger N, Hatam I (2009) Fungal apoptosis: function, genes and gene function. FEMS Microbiol Rev 33:833–854. https://doi.org/10.1111/j.1574-6976.2009.00180.x
Singh S, Ambastha V, Levine A, Sopory SK, Yadava PK, Tripathy BC, Tiwari BS (2015) Anhydrobiosis and programmed cell death in plants: commonalities and differences. Curr Plant Biol 2:12–20. https://doi.org/10.1016/j.cpb.2014.12.001
Sirisha VL, Sinha M, D’Souza JS (2014) Menadione-induced caspase-dependent programmed cell death in the green chlorophyte Chlamydomonas reinhardtii. J Phycol 50:587–601. https://doi.org/10.1111/jpy.12188
Snyder CM, Shroff EH, Liu J, Chandel NS (2009) Nitric oxide induces cell death by regulating anti-apoptotic BCL-2 family members. PLoS One 4. https://doi.org/10.1371/journal.pone.0007059
Ünal D, Uyanikgil Y (2011) UV-B induces cell death in the lichen Physcia semipinnata (J.F.Gmel). Turkish J Biol 35:137–144. https://doi.org/10.3906/biy-0901-5
Van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10:117–122. https://doi.org/10.1016/j.tplants.2005.01.006
Vavilala SL, Gawde KK, Sinha M, D’Souza JS (2015) Programmed cell death is induced by hydrogen peroxide but not by excessive ionic stress of sodium chloride in the unicellular green alga Chlamydomonas reinhardtii. Eur J Phycol 50:422–438. https://doi.org/10.1080/09670262.2015.1070437
Virginio C, MacKenzie A, North RA, Surprenant A (1999) Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol 519:335–346
Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6:177–183
Wlodkowic D, Khoshmanesh K, Sharpe JC, Darzynkiewicz Z, Cooper JM (2011) Apoptosis goes on a chip: advances in the microfluidic analysis of programmed cell death. Anal Chem 83:6439–6446
Yordanova ZP, Woltering EJ, Kapchina-Toteva VM, Iakimova ET (2013) Mastoparan-induced programmed cell death in the unicellular alga Chlamydomonas reinhardtii. Ann Bot 111:191–205
Yu YTN, Snyder L (1994) Translation elongation factor Tu cleaved by a phage-exclusion system. Proc Natl Acad Sci U S A 91:802–806. https://doi.org/10.1073/pnas.91.2.802
Zuppini A, Andreoli C, Baldan B (2007) Heat stress: an inducer of programmed cell death in Chlorella saccharophila. Plant Cell Physiol 48:1000–1009. https://doi.org/10.1093/pcp/pcm070
Acknowledgements
A special thanks is dedicated to Prof. Eva Barreno for her continuous example, inspiration and support in the elucidation of the cell biology of lichens and phycobionts.
Funding
This study was funded by MINECO (CGL2016–79158-P), FEDER, YEI-CAM (PEJ-2017-AI/AMB-6337) and the Generalitat Valenciana, GVA Excellence in Research (PROMETEO III /2017/039).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Expósito, J.R., Mejuto, I. & Catalá, M. Detection of active cell death markers in rehydrated lichen thalli and the involvement of nitrogen monoxide (NO).. Symbiosis 82, 59–67 (2020). https://doi.org/10.1007/s13199-020-00727-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-020-00727-3
Keywords
Profiles
- Myriam Catalá View author profile