Abstract
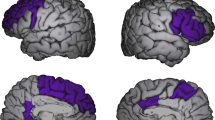
Pathological disturbances of mood may follow a 'bipolar' course, in which normal moods alternate with both depression and mania, or a 'unipolar' course, in which only depression occurs1–3. Both bipolar and unipolar disorders can be heritable illnesses associated with neurochemical, neuroendocrine and autonomic abnormalities. The neurobiological basis for these abnormalities has not been established2,3. Using positron emission tomographic (PET) images of cerebral blood flow and rate of glucose metabolism to measure brain activity, we have now localized an area of abnormally decreased activity in the pre-frontal cortex ventral to the genu of the corpus callosum in both familial bipolar depressives and familial unipolar depressives. This decrement in activity was at least partly explained by a corresponding reduction in cortical volume4, as magnetic resonance imaging (MRI) demonstrated reductions in the mean grey matter volume in the same area of 39 and 48% in the bipolar and unipolar samples, respectively. This region has previously been implicated in the mediation of emotional and autonomic responses to socially significant or provocative stimuli, and in the modulation of the neurotransmitter systems targeted by antidepressant drugs3,5–10.
This is a preview of subscription content, access via your institution
Access options
Access to this article via Institution of Civil Engineers Library is not available.
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) (American Psychiatric Association, Washington, DC, 1987).
Goodwin, F. K. & Jamison, K. R. Manic-depressive Illness (Oxford University Press, New York, 1990).
Drevets, W. C. & Todd, R. D. in Adult Psychiatry (ed. Guze, S. B.) 99–142 (Mosby, St Louis, 1997).
Mazziotta, J. C., Phelps, M. E., Plummer, D. & Kuhl, D. E. Quantitation in positron emission computed tomography: 5. Physical-anatomical effects. J. Comput. Assist. Tomogr. 5, 734–743 (1981).
Carmichael, S. T. & Price, J. L. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615–641 (1995).
Neafsey, E. J., Terreberry, R. R., Hurley, K. M., Ruit, K. G. & Frysztak, R. J. in Neurobiology of Cingulate Cortex and Limbic Thalamus (eds Vogt, B. A. & Gabriel, M.) 206–223 (Birkhauser, Boston, 1993).
Sesack, S. R., Deutch, A. Y., Roth, R. H. & Bunney, B. S. Topographic organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study using Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213–242 (1989).
Damasio, A. R., Tranel, D. & Damasio, H. Individuals with sociopathic behavior caused by frontal damage fail to respond automatically to social stimuli. Behav. Brain Res. 41, 81–94 (1990).
Bechara, A., Tranel, D., Damasio, H. & Damasio, A. R. Failure to respond autonomically to anticipated future outcomes following damage to the prefrontal cortex. Cerebr. Corf. 6, 215–225 (1996).
Damasio, A. R. Descarte's Error: Emotion, Reason, and the Human Brain. (Grosset/Putnam, New York, 1994; Picador MacMillan, London, 1995).
Drevets, W. C. et al. A functional anatomical study of unipolar depression. J. Neurosci. 12, 3628–3641 (1992).
Winokur, G. The development and validity of familial subtypes in primary unipolar depression. Pharmacopsychiatry 15, 142–146 (1982).
Baxter, L. R. et al. Cerebral metabolic rates for glucose in mood disorders. Arch. Gen. Psychiat. 42, 441–447 (1985).
Baxter, L. R. et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiat. 46, 243–250 (1989).
Buchsbaum, M. S. et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J. Affect. Dis. 10, 137–152 (1986).
Cohen, R. M. et al. Evidence for common alterations in cerebral glucose metabolism in major affective disorders and schizophrenia. Nauropsychopharmacology 2, 241–254 (1989).
Dolan, R. J. et al. Dorsolateral prefrontal cortex dysfunction in the major psychoses: symptom or disease specificity? J. Neurol. Neurosurg. Psychiat. 56, 1290–1294 (1993).
Drevets, W. C. & Botteron, K. in Adult Psychiatry (ed. Guze, S. B.) 53–82 (Mosby, St Louis, 1996).
Drevets, W. C. & Raichle, M. E. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognit. Emot. (in the press).
Drevets, W. C., Spitznagel, E. L., MacLeod, A. K. & Raichle, M. E. Discriminatory capability of PET measurements of regional blood flow in familial pure depressive disease. Abstr. Soc. Neurosci. 18, 1596 (1992).
Young, R. C., Biggs, J. T., Ziegler, V. E. & Meyer, D. A. A rating scale for mania: reliability, validity and sensitivity. Brit. J. Psychiat. 133, 429–435 (1978).
DiRocco, R. J., Kageyama, G. H. & Wong-Riley, M. T. The relationship between CNS metabolism and cytoarchitecture: a review of 14C-deoxyglucose studies with correlation to cytochrome oxidase histochemistry. Comp. Med. Imag. Graph. 13, 81–92 (1989).
Pearlson, G. D. et al. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol. Psychiat. 41, 1–14 (1997).
Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiat. 23, 56–62 (1960).
Raichle, M. E., Martin, W. R. W., Herscovitch, P., Mintun, M. A. & Markham, J. Brain blood flow measured with intravenous 218O. II. Implementation and validation. J. Nucl. Med. 24, 790–798 (1983).
Fox, P. T. & Mintun, M. A. Noninvasive functional brain mapping by change distribution analysis of averaged PET images of H215O tissue activity. J. Nucl. Med. 30, 141–149 (1989).
Phelps, M. E. et al. Tomographic measurements of local cerebral glucose metabolic rate in humans with [F-18]2-fluoro-2-deoxy-D-glucose: validation of method. Ann. Neurol. 6, 371–388 (1979).
Talairach, J. & Tournoux, P. in Co-Planar Stereotaxic Atlas of the Human Brain 1–122 (Theime, Stuttgart, 1988).
Fox, P. T., Perlmutter, J. S. & Raichle, M. E. A stereotactic method of anatomical localization for positron emission tomography. J. Comput. Assist. Tomogr. 9, 141–153 (1985).
Carmichael, S. T. & Price, J. L. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. J. Comp. Neurol. 346, 366–402 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Drevets, W., Price, J., Simpson, J. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827 (1997). https://doi.org/10.1038/386824a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/386824a0