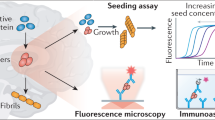
Abstract
Several sporadic and genetic diseases are caused by protein misfolding. These include cystic fibrosis and other devastating diseases of childhood as well as Alzheimer's, Parkinson's and other debilitating maladies of the elderly. A unified view of the molecular and cellular pathogenesis of these conditions has led to the search for chemical chaperones that can slow, arrest or revert disease progression. Molecules are now emerging that link our biophysical insights with our therapeutic aspirations.
This is a preview of subscription content, access via your institution
Access options
Access to this article via Institution of Civil Engineers Library is not available.
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Anfinsen, C. B. Principles that govern the folding of protein chains. Science 181, 223–230 (1973).
Powell, K. & Zeitlin, P. L. Therapeutic approaches to repair defects in ΔF508 CFTR folding and cellular targeting. Adv. Drug Deliv. Rev. 54, 1395–1408 (2002).
Howard, M. & Welch, W. J. Manipulating the folding pathway of ΔF508 CFTR using chemical chaperones. Methods Mol. Med. 70, 267–275 (2002).
Carrell, R. W. & Lomas, D. A. Mechanisms of disease. Alpha1-antitrypsin deficiency—a model for conformational diseases. N. Engl. J. Med. 346, 45–53 (2002).
Rochet, J.-C. & Lansbury, P. T. Jr. Amyloid fibrillogenesis: themes and variations. Curr. Opin. Struct. Biol. 10, 60–68 (2000).
Kelly, J. W. Alternative conformations of amyloidogenic proteins govern their behavior. Curr. Opin. Struct. Biol. 6, 11–17 (1996).
Fan, J.-Q. A contradictory treatment for lysosomal storage disorders: inhibitors enhance mutant enzyme activity. Trends Pharm. Sci. 24, 355–360 (2003).
Sawkar, A. R. et al. Chemical chaperones increase the cellular activity of N370S β-glucosidase: a therapeutic strategy for Gaucher disease. Proc. Natl Acad. Sci. USA 99, 15428–15433 (2002).
Morello, J.-P. et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J. Clin. Inv. 105, 887–895 (2000).
Petaja-Repo, U. E. et al. Ligands act as pharmacological chaperones and increase the efficiency of δ opioid receptor maturation. EMBO J. 21, 1628–1637 (2002).
Janovick, J. A. et al. Structure–activity relations of successful pharmacologic chaperones for rescue of naturally occurring and manufactured mutants of the gonadotropin-releasing hormone receptor. J. Pharm. Exp. Ther. 305, 608–614 (2003).
Noorwez, S. M. et al. Pharmacological chaperone-mediated in vivo folding and stabilization of the P23H-opsin mutant associated with autosomal dominant retinitis pigmentosa. J. Biol. Chem. 278, 14442–14450 (2003).
Ficker, E., Obejero-Paz, C. A., Zhao, S. & Brown, A. M. The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J. Biol. Chem. 277, 4989–4998 (2002).
Fan, J.-Q., Ishii, S., Asano, N. & Suzuki, Y. Accelerated transport and maturation of lysosomal α-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nature Med. 5, 112–115 (1999).
Frustaci, A. et al. Improvement in cardiac function in the cardiac variant of Fabry's disease with galactose-infusion therapy. N. Engl. J. Med. 345, 25–32 (2001).
Springsteel, M. F. et al. Benzoflavone activators of the cystic fibrosis transmembrane conductance regulator: towards a pharmacophore model for the nucleotide-binding ___domain. Bioorg. Medic. Chem. 11, 4113–4120 (2003).
Burrows, J. A. J., Willis, L. K. & Perlmutter, D. H. Chemical chaperones mediate increased secretion of mutant α1-antitrypsin (α1-AT) Z: a potential pharmacological strategy for prevention of liver injury and emphysema in α1-AT deficiency. Proc. Natl Acad. Sci. USA 97, 1796–1801 (2000).
Hammarstrom, P., Jiang, X., Hurshman, A. R., Powers, E. T. & Kelly, J. W. Sequence-dependent denaturation energetics: a major determinant in amyloid disease diversity. Proc. Natl Acad. Sci. USA 99, 16427–16432 (2002).
Hammarstrom, P., Wiseman, R. L., Powers, E. T. & Kelly, J. W. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science 299, 713–716 (2003).
Schneider, F., Hammarstrom, P. & Kelly, J. W. Transthyretin slowly exchanges subunits under physiological conditions: a convenient chromatographic method to study subunit exchange in oligomeric proteins. Protein Sci. 10, 1606–1613 (2001).
Nikolova, P. V., Wong, K.-B., DeDecker, B., Henckel, J. & Fersht, A. R. Mechanism of rescue of common p53 cancer mutations by second-site suppressor mutations. EMBO J. 19, 370–378 (2000).
Sacchettini, J. C. & Kelly, J. W. Therapeutic strategies for human amyloid diseases. Nature Rev. Drug Discov. 1, 267–275 (2002).
White, J. T. & Kelly, J. W. Support for the multigenic hypothesis of amyloidosis: the binding stoichiometry of retinol-binding protein, vitamin A, and thyroid hormone influences transthyretin amyloidogenicity in vitro. Proc. Natl Acad. Sci. USA 98, 13019–13024 (2001).
Korth, C., May, B. C. H., Cohen, F. E. & Prusiner, S. B. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl Acad. Sci. USA 98, 9836–9841 (2001).
May, B. C. H. et al. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc. Natl Acad. Sci. USA 100, 3416–3421 (2003).
Vogtherr, M. et al. Antimalarial drug quinacrine binds to C-terminal helix of cellular prion protein. J. Med. Chem. 46, 3563–3564 (2003).
Peretz, D. et al. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412, 739–743 (2001).
White, A. R. et al. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature 422, 80–83 (2003).
Heppner, F. L. et al. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 294, 178–182 (2001).
Solomon, B. Anti-aggregating antibodies, a new approach towards treatment of conformational diseases. Curr. Med. Chem. 9, 1737–1749 (2002).
Telling, G. C. et al. Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell 83, 79–90 (1995).
Ohno, S. et al. The antisense approach in amyloid light chain amyloidosis: identification of monoclonal Ig and inhibition of its production by antisense oligonucleotides in in vitro and in vivo models. J. Immunol. 169, 4039–4045 (2002).
Findeis, M. A. Peptide inhibitors of beta amyloid aggregation. Curr. Top. Med. Chem. 2, 417–423 (2002).
Yang, D.-S. et al. Assembly of Alzheimer's amyloid-β fibrils and approaches for therapeutic intervention. Amyloid 8, 10–19 (2001).
Howlett, D. R. et al. Inhibition of fibril formation in β-amyloid peptide by a novel series of benzofurans. Biochem. J. 340, 283–289 (1999).
Kelly, J. W. & Balch, W. E. Amyloid as a natural product. J. Cell Biol. 161, 461–462 (2003).
Lamber, M. P. et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl Acad. Sci. USA 95, 6448–6453 (1998).
Kayed, R. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300, 486–489 (2003).
Walsh, D. M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Wolfe, M. S. γ-Secretase as a target for Alzheimer's disease. Curr. Top. Med. Chem. 2, 371–383 (2002).
Vassar, R. β-Secretase (BACE) as a drug target for Alzheimer's disease. Adv. Drug Deliv. Rev. 54, 1589–1602 (2002).
Jarrett, J. T. & Lansbury, P. T. Jr. Seeding 'one-dimensional crystallization' of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73, 1055–1058 (1993).
Golde, T. E., Eriksen, J. L., Weggen, S., Sagi, S. A. & Koo, E. H. Nonsteroidal antiinflammatory drugs as therapeutic agents for Alzheimer's disease. Drug Dev. Res. 56, 415–420 (2002).
Spooner, E. T., Desai, R. V., Mori, C., Leverone, J. F. & Lemere, C. A. The generation and characterization of potentially therapeutic Aβ antibodies in mice: differences according to strain and immunization protocol. Vaccine 21, 290–297 (2002).
Nicoll, J. A. R. et al. Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nature Med. 9, 448–452 (2003).
Solomon, B. Protective molecules in Alzheimer's disease: therapeutic antibodies. Drug News Perspect. 15, 410–416 (2002).
Colon, W. & Kelly, J. W. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry 31, 8654–8660 (1992).
Fandrich, M., Fletcher, M. A. & Dobson, C. M. Amyloid fibrils from muscle myoglobin. Nature 410, 165–166 (2001).
Baskakov, I. V., Legname, G., Prusiner, S. B. & Cohen, F. E. Folding of prion protein to its native α-helical conformation is under kinetic control. J. Biol. Chem. 276, 19687–19690 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cohen, F., Kelly, J. Therapeutic approaches to protein-misfolding diseases. Nature 426, 905–909 (2003). https://doi.org/10.1038/nature02265
Issue Date:
DOI: https://doi.org/10.1038/nature02265
This article is cited by
-
Efficient inhibition of amyloid fibrillation and cytotoxicity of α-synuclein and human insulin using biosynthesized silver nanoparticles decorated by green tea polyphenols
Scientific Reports (2024)
-
Topological data analysis gives two folding paths in HP35(nle-nle), double mutant of villin headpiece subdomain
Scientific Reports (2022)
-
Drug resistance: from bacteria to cancer
Molecular Biomedicine (2021)
-
Therapeutic targeting of the PI4K2A/PKR lysosome network is critical for misfolded protein clearance and survival in cancer cells
Oncogene (2020)
-
Copper Ion Uptake by Chitosan in the Presence of Amyloid-β and Histidine
Applied Biochemistry and Biotechnology (2020)