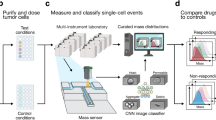
Abstract
Assays that can determine the response of tumor cells to cancer therapeutics could greatly aid the selection of drug regimens for individual patients. However, the utility of current functional assays is limited, and predictive genetic biomarkers are available for only a small fraction of cancer therapies. We found that the single-cell mass accumulation rate (MAR), profiled over many hours with a suspended microchannel resonator, accurately defined the drug sensitivity or resistance of glioblastoma and B-cell acute lymphocytic leukemia cells. MAR revealed heterogeneity in drug sensitivity not only between different tumors, but also within individual tumors and tumor-derived cell lines. MAR measurement predicted drug response using samples as small as 25 μl of peripheral blood while maintaining cell viability and compatibility with downstream characterization. MAR measurement is a promising approach for directly assaying single-cell therapeutic responses and for identifying cellular subpopulations with phenotypic resistance in heterogeneous tumors.
This is a preview of subscription content, access via your institution
Access options
Access to this article via ICE Institution of Civil Engineers is not available.
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mellinghoff, I.K. et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N. Engl. J. Med. 353, 2012–2024 (2005).
Sos, M.L. et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J. Clin. Invest. 119, 1727–1740 (2009).
Garraway, L.A. & Jänne, P.A. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2, 214–226 (2012).
Klempner, S.J., Myers, A.P. & Cantley, L.C. What a tangled web we weave: emerging resistance mechanisms to inhibition of the phosphoinositide 3-kinase pathway. Cancer Discov. 3, 1345–1354 (2013).
Haibe-Kains, B. et al. Inconsistency in large pharmacogenomic studies. Nature 504, 389–393 (2013).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Francis, J.M. et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 4, 956–971 (2014).
Burstein, H.J. et al. American Society of Clinical Oncology clinical practice guideline update on the use of chemotherapy sensitivity and resistance assays. J. Clin. Oncol. 29, 3328–3330 (2011).
Friedman, A.A., Letai, A., Fisher, D.E. & Flaherty, K.T. Precision medicine for cancer with next-generation functional diagnostics. Nat. Rev. Cancer 15, 747–756 (2015).
Crystal, A.S. et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 346, 1480–1486 (2014).
Burg, T.P. et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 446, 1066–1069 (2007).
Godin, M. et al. Using buoyant mass to measure the growth of single cells. Nat. Methods 7, 387–390 (2010).
Son, S. et al. Direct observation of mammalian cell growth and size regulation. Nat. Methods 9, 910–912 (2012).
Byun, S., Hecht, V.C. & Manalis, S.R. Characterizing Cellular Biophysical Responses to Stress by Relating Density, Deformability, and Size. Biophys. J. 109, 1565–1573 (2015).
Wu, S. et al. Quantification of cell viability and rapid screening anti-cancer drug utilizing nanomechanical fluctuation. Biosens. Bioelectron. 77, 164–173 (2016).
Lathia, J.D. et al. Direct in vivo evidence for tumor propagation by glioblastoma cancer stem cells. PLoS One 6, e24807 (2011).
Deleyrolle, L.P. et al. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain 134, 1331–1343 (2011).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Pui, C.H., Relling, M.V. & Downing, J.R. Acute lymphoblastic leukemia. N. Engl. J. Med. 350, 1535–1548 (2004).
Cortes, J.E. et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 367, 2075–2088 (2012).
Verreault, M. et al. Preclinical efficacy of the MDM2 inhibitor RG7112 in MDM2-amplified and TP53 wild-type glioblastomas. Clin. Cancer Res. 22, 1185–1196 (2015).
Jeay, S. et al. A distinct p53 target gene set predicts for response to the selective p53-HDM2 inhibitor NVP-CGM097. eLife 4 http://dx.doi.org/10.7554/eLife.06498 (published online 12 May 2015).
Andreeff, M. et al. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin. Cancer Res. 22, 868–876 (2016).
Lane, A.A. et al. Triplication of a 21q22 region contributes to B cell transformation through HMGN1 overexpression and loss of histone H3 Lys27 trimethylation. Nat. Genet. 46, 618–623 (2014).
Pencina, M.J., D'Agostino, R.B. Sr., D'Agostino, R.B. Jr. & Vasan, R.S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 27, 157–172, discussion 207–212 (2008).
Cermak, N. et al. High-throughput growth measurements on single cells via serial microfluidic mass sensor arrays. Nat. Biotechnol. http://dx.doi.org/10.1038/nbt.3666 (2016).
Fischer, T. et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J. Clin. Oncol. 28, 4339–4345 (2010).
Shalek, A.K. et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature 510, 363–369 (2014).
Montero, J. et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 160, 977–989 (2015).
Jonas, O. et al. An implantable microdevice to perform high-throughput in vivo drug sensitivity testing in tumors. Sci. Transl. Med. 7, 284ra57 (2015).
Klco, J.M. et al. Genomic impact of transient low-dose decitabine treatment on primary AML cells. Blood 121, 1633–1643 (2013).
Acknowledgements
These studies were supported by R01 CA170592 (S.R.M., K.L.L., P.Y.W.), P50 CA165962 (K.L.L., P.Y.W.), P01 CA142536 (K.L.L.) and R33 CA191143 (S.R.M., D.M.W.) from the US National Institutes of Health, U54 CA143874 from the National Cancer Institute (S.R.M.), and partially by Cancer Center Support (core) Grant P30 CA14051 from the National Cancer Institute, The Bridge Project, a partnership between the Koch Institute for Integrative Cancer Research at MIT and the Dana-Farber/Harvard Cancer Center (DF/HCC) (S.R.M., D.M.W.), and the Dana-Farber Cancer Institute Brain Tumor Therapeutics Accelerator Program (P.Y.W., K.L.L.). A.I. acknowledges support from Fondation ARC pour la Recherche sur le Cancer, The Institut Universitaire de Cancérologie, OncoNeuroThèque and the program Investissements d'avenir” ANR-10-IAIHU-06. M.M.S. acknowledges support from the NIH/NIGMS T32 GM008334, Interdepartmental Biotechnology Training Program grant. N. Chou acknowledges support from the National Science Scholarship, Agency for Science, Technology and Research (STAR), Singapore. D.M.W. is a Leukemia and Lymphoma Scholar. M.A.M. gratefully acknowledges support from the institutional research training grant T32 CA009172, from the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
N. Cermak, S.O. and S.R.M. designed devices. M.M.S., N. Chou, N. Cermak and S.O. designed and constructed the experimental setup. C.L.M., D.S.K., S.H., A.I., P.Y.W. and K.L.L. managed and created BT GBM-PDCLs. M.A.M. and H.L. managed and processed murine models of B-cell acute lymphocytic leukemia. M.A.M., H.L. and N.A.C. procured and processed patient samples. M.M.S., C.L.M., N. Chou, M.A.M., D.S.K., Y.K., N.L.C., N.A.C., N. Cermak, D.M.W., K.L.L. and S.R.M. designed the experiments. M.M.S., C.L.M., N. Chou, M.A.M., D.S.K., Y.K., R.J.K., H.L., S.H., N.L.C. and N.A.C. performed the experiments. M.M.S., C.L.M., N. Chou., M.A.M., D.S.K., Y.K., R.J.K., N.L.C., N. Cermak, N.A.C. analyzed the data. M.M.S., C.L.M., N. Chou, D.M.W., K.L.L. and S.R.M. wrote the paper with input from all of the other authors.
Corresponding authors
Ethics declarations
Competing interests
S.R.M. is a cofounder of Affinity Biosensors, which develops techniques relevant to the research presented. S.O. and M.M.S. anticipate employment at Affinity Biosensors. D.M.W. is a consultant for and receives research support from Novartis.
Integrated supplementary information
Supplementary Figure 1 Sphere forming assay of BT145 GBM PDCL
(a) Sphere forming potential of single BT145 GBM cells isolated from bulk culture or SMR post-MAR measurement. p-values reflect output of Pearson’s chi-squared test. (b) Representative single-cell trajectories paired with images of sphere forming potential 2-weeks after measurement. Note that even cells with minimal or negative growth over the 15-minute period may retain tumorsphere-forming potential (bottom right).
Supplementary Figure 2 Growth heterogeneity maintained across multiple passages of PCDLs
Representative staining results from immunohistochemistry for Ki67 on BT145, BT159, BT179, BT240, BT320, and BT333 cell lines. Results quantified as percentage of total cells stained positive.
Supplementary Figure 3 Growth heterogeneity maintained across multiple passages of PCDLs
Box plot comparison of MAR normalized to mass for the same cell lines as in Figure 1c with three passages of GBM-PDCLs shown separately. Boxes represent the inter-quartile range and white squares the average of all measurements. From left to right, n = 84; 46; 13, 14, 17; 12, 21, 18; 21, 18, 13; 19, 21, 21; 16, 16, 16; 14, 18, 14; 18, 25, 21; 19, 22, 18.
Supplementary Figure 4 PDCLs following treatment with MDM2 inhibitor RG7112
Scatter plots of MAR versus mass for BT484, BT3731, BT159, BT333 cells following treatment with 1 μM RG7112.
Supplementary Figure 5 Dose-response curves for GBM PDCLs treated with RG7112
Curves from PDCLs were generated using CellTiter-Glo at 72 hrs, following treatment with 1 μM RG7112. IC50 values embedded in each graph reflect the output of a four parameter nonlinear regression model +/- the range of the 95% confidence interval.
Supplementary Figure 6 Viability and purity of primary murine B-ALL by flow cytometry
(a) Representative dot plots of cells stained with DAPI and Annexin V, as markers of viability. (b) Representative histogram of GFP expression after cell sorting. Leukemia cells in this model uniquely express GFP. FACS analysis was performed on all primary murine splenocyte samples.
Supplementary Figure 7 In vivo clonal dynamics in a transgenic murine model of BCR-ABL B-ALL
(a) The allelic frequency of BCR-ABL T315I in two mice treated with nilotinib, a surrogate for imatinib. Allelic frequencies were calculated by visual measurement of the relative heights of the electropherogram peaks; values from the paired forward and reverse sequencing phases were averaged to produce the allelic frequencies shown. Mouse 1 (closed circles) was sacrificed on day 14 for routine pharmacodynamic assessment (open circle). Mouse 2 (closed squares) was sacrificed after developing clinical signs of advanced leukemia on day 33 (open square). (b) Representative electropherograms showing ABL codon 315 (in gray; ACT indicates wild type T315, and ATT indicates the point mutation T315I). As demonstrated, the mutant subclone expands in relation to WT during treatment with nilotinib, to which WT but not T315I BCR-ABL is sensitive.
Supplementary Figure 8 MAR or mass can be used individually as a classifier for drug susceptibility
Primary murine BCR-ABL ALL and BCR-ABL T315I ALL cells treated with 1 μM imatinib, or 100 nM ponatinib, respectively. (a) MAR versus mass plot with overlay of an orthogonal vector (dotted line) designating the threshold resulting from LDA. Cells treated with drug are in red, and DMSO control cells are blue (b) ROC curves from same paired control and treatment data following LDA of MAR per mass plot. (c) Overlaid ROC curves of paired control and treatment data for all treatment replicates using only mass or MAR parameter. Cells treated with therapy to which they are sensitive or resistant are shown with blue solid lines or red dotted lines, respectively.
Supplementary Figure 9 Predictive power of MAR for cells isolated from circulation
Primary murine BCR-ABL T315I cells isolated from circulation, treated with DMSO or 100 nM ponatinib. ROC curves of paired control and treatment data for each replicate following LDA.
Supplementary Figure 10 Patient sample treated in vivo shows consistent reduction in MAR
MAR versus Mass plot for blasts from peripheral blood samples of AML. Pre-treatment sample shown in black (n=86), and sample obtained after the patient received 48 hrs of of treatment with an experimental MDM2 inhibitor shown in red (n=95).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–10 and Supplementary Table 1 (PDF 1706 kb)
Rights and permissions
About this article
Cite this article
Stevens, M., Maire, C., Chou, N. et al. Drug sensitivity of single cancer cells is predicted by changes in mass accumulation rate. Nat Biotechnol 34, 1161–1167 (2016). https://doi.org/10.1038/nbt.3697
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3697