Abstract
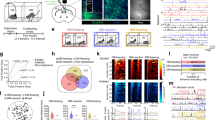
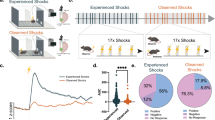
Fear can be acquired vicariously through social observation of others suffering from aversive stimuli. We found that mice (observers) developed freezing behavior by observing other mice (demonstrators) receive repetitive foot shocks. Observers had higher fear responses when demonstrators were socially related to themselves, such as siblings or mating partners. Inactivation of anterior cingulate cortex (ACC) and parafascicular or mediodorsal thalamic nuclei, which comprise the medial pain system representing pain affection, substantially impaired this observational fear learning, whereas inactivation of sensory thalamic nuclei had no effect. The ACC neuronal activities were increased and synchronized with those of the lateral amygdala at theta rhythm frequency during this learning. Furthermore, an ACC-limited deletion of Cav1.2 Ca2+ channels in mice impaired observational fear learning and reduced behavioral pain responses. These results demonstrate the functional involvement of the affective pain system and Cav1.2 channels of the ACC in observational social fear.
This is a preview of subscription content, access via your institution
Access options
Access to this article via ICE Institution of Civil Engineers is not available.
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Olsson, A. & Phelps, E.A. Social learning of fear. Nat. Neurosci. 10, 1095–1102 (2007).
LeDoux, J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Phelps, E.A. & LeDoux, J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187 (2005).
Adolphs, R. Cognitive neuroscience of human social behavior. Nat. Rev. Neurosci. 4, 165–178 (2003).
Frith, C.D. Social cognition. Phil. Trans. R. Soc. Lond. B 363, 2033–2039 (2008).
Hooker, C.I., Germine, L.T., Knight, R.T. & D'Esposito, M. Amygdala response to facial expressions reflects emotional learning. J. Neurosci. 26, 8915–8922 (2006).
Mineka, S. & Cook, M. Mechanisms involved in the observational conditioning of fear. J. Exp. Psychol. Gen. 122, 23–38 (1993).
Olsson, A., Nearing, K.I. & Phelps, E.A. Learning fears by observing others: the neural systems of social fear transmission. Soc. Cogn. Affect. Neurosci. 2, 3–11 (2007).
Olsson, A. & Phelps, E.A. Learned fear of “unseen” faces after Pavlovian, observational, and instructed fear. Psychol. Sci. 15, 822–828 (2004).
Adolphs, R. et al. A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72 (2005).
Whalen, P.J. et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 18, 411–418 (1998).
Miller, R.E., Murphy, J.V. & Mirsky, I.A. Non-verbal communication of affect. J. Clin. Psychol. 15, 155–158 (1959).
Rice, G.E. & Gainer, P. “Altruism” in the albino rat. J. Comp. Physiol. Psychol. 55, 123–125 (1962).
Church, R.M. Emotional reactions of rats to the pain of others. J. Comp. Physiol. Psychol. 52, 132–134 (1959).
Chen, Q., Panksepp, J.B. & Lahvis, G.P. Empathy is moderated by genetic background in mice. PLoS One 4, e4387 (2009).
Price, D.D. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772 (2000).
Vogt, B.A. Pain and emotion interactions in subregions of the cingulate gyrus. Nat. Rev. Neurosci. 6, 533–544 (2005).
Bush, G., Luu, P. & Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 4, 215–222 (2000).
Devinsky, O., Morrell, M.J. & Vogt, B.A. Contributions of anterior cingulate cortex to behavior. Brain 118, 279–306 (1995).
Morrison, I. & Downing, P.E. Organization of felt and seen pain responses in anterior cingulate cortex. Neuroimage 37, 642–651 (2007).
Gao, Y.J., Ren, W.H., Zhang, Y.Q. & Zhao, Z.Q. Contributions of the anterior cingulate cortex and amygdala to pain- and fear-conditioned place avoidance in rats. Pain 110, 343–353 (2004).
Johansen, J.P., Fields, H.L. & Manning, B.H. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA 98, 8077–8082 (2001).
LaGraize, S.C. et al. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp. Neurol. 188, 139–148 (2004).
Jackson, P.L., Meltzoff, A.N. & Decety, J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage 24, 771–779 (2005).
Singer, T. et al. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004).
Apkarian, A.V. Pain perception in relation to emotional learning. Curr. Opin. Neurobiol. 18, 464–468 (2008).
Auvray, M., Myin, E. & Spence, C. The sensory-discriminative and affective-motivational aspects of pain. Neurosci. Biobehav. Rev. 34, 214–223 (2010).
Day, M. et al. Stimulation of 5-HT(2) receptors in prefrontal pyramidal neurons inhibits Cav1.2 L-type Ca2+ currents via a PLCβ/IP3/calcineurin signaling cascade. J. Neurophysiol. 87, 2490–2504 (2002).
Liauw, J., Wu, L.J. & Zhuo, M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J. Neurophysiol. 94, 878–882 (2005).
Meredith, R.M. et al. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron 54, 627–638 (2007).
Moosmang, S. et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor–independent synaptic plasticity and spatial memory. J. Neurosci. 25, 9883–9892 (2005).
Wiltgen, B.J. & Silva, A.J. Memory for context becomes less specific with time. Learn. Mem. 14, 313–317 (2007).
Lee, H.J., Choi, J.S., Brown, T.H. & Kim, J.J. Amygdalar NMDA receptors are critical for the expression of multiple conditioned fear responses. J. Neurosci. 21, 4116–4124 (2001).
Bissière, S. et al. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biol. Psychiatry 63, 821–831 (2008).
Seidenbecher, T., Laxmi, T.R., Stork, O. & Pape, H.C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301, 846–850 (2003).
Jo, D. et al. Epigenetic regulation of gene structure and function with a cell-permeable Cre recombinase. Nat. Biotechnol. 19, 929–933 (2001).
Millan, M.J. The induction of pain: an integrative review. Prog. Neurobiol. 57, 1–164 (1999).
Han, C.J. et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA 100, 13087–13092 (2003).
Johansen, J.P. & Fields, H.L. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 7, 398–403 (2004).
Malin, E.L. & McGaugh, J.L. Differential involvement of the hippocampus, anterior cingulate cortex and basolateral amygdala in memory for context and footshock. Proc. Natl. Acad. Sci. USA 103, 1959–1963 (2006).
Singer, W. Neuronal synchrony: a versatile code for the definition of relations? Neuron 24, 49–65, 111–125 (1999).
Varela, F., Lachaux, J.P., Rodriguez, E. & Martinerie, J. The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239 (2001).
Raghavachari, S. et al. Theta oscillations in human cortex during a working-memory task: evidence for local generators. J. Neurophysiol. 95, 1630–1638 (2006).
Sarnthein, J. et al. Synchronization between prefrontal and posterior association cortex during human working memory. Proc. Natl. Acad. Sci. USA 95, 7092–7096 (1998).
Mizuhara, H. & Yamaguchi, Y. Human cortical circuits for central executive function emerge by theta phase synchronization. Neuroimage 36, 232–244 (2007).
Preston, S.D. & de Waal, F.B. Empathy: its ultimate and proximate bases. Behav. Brain Sci. 25, 1–20, discussion 20–71 (2002).
Hoffman, M.L. Empathy, its development and prosocial implications. Nebr. Symp. Motiv. 25, 169–217 (1977).
Langford, D.J. et al. Social modulation of pain as evidence for empathy in mice. Science 312, 1967–1970 (2006).
Phillips, M.L., Drevets, W.C., Rauch, S.L. & Lane, R. Neurobiology of emotion perception II. Implications for major psychiatric disorders. Biol. Psychiatry 54, 515–528 (2003).
Seisenberger, C. et al. Functional embryonic cardiomyocytes after disruption of the L-type alpha1C (Cav1.2) calcium channel gene in the mouse. J. Biol. Chem. 275, 39193–39199 (2000).
Acknowledgements
We thank K. Lee for help with Matlab analysis. This work was supported by the National Honor Scientist program of Korea and the Center of Excellence program from the Korea Institute of Science and Technology.
Author information
Authors and Affiliations
Contributions
D. Jeon and H.-S.S. designed the experiments. D. Jeon purified Cre protein. D. Jo and H.E.R. made and provided the vector containing His6-NLS-Cre-MTS. D. Jeon, S.K. and M.C. performed surgeries, microinjections and immunostainings and analyzed the data. D. Jeon and S.K. performed in vivo electrophysiology. S.-Y.L., D.R. and J.-P. K. generated the Cav1.2 conditional mice. D. Jeon and H.-S.S. wrote the manuscript. All of the authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–11 and Supplementary Discussion (PDF 618 kb)
Rights and permissions
About this article
Cite this article
Jeon, D., Kim, S., Chetana, M. et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci 13, 482–488 (2010). https://doi.org/10.1038/nn.2504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2504