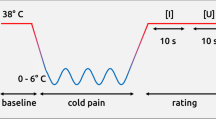
Key Points
-
The cingulate gyrus participates in pain and emotion processing. Although Brodmann identified two regions without knowledge of their functional activity, recent cytoarchitectural, connection and functional imaging studies show that the cingulate gyrus actually has four regions, with associated subregions, and that each makes a qualitatively unique contribution to brain functions. These regions and subregions are the subgenual and pregenual anterior cingulate cortex (sACC and pACC), the anterior and posterior midcingulate cortex (aMCC and pMCC), the dorsal and ventral posterior cingulate cortex (dPCC and vPCC), and the retrosplenial cortex (RSC). Pain processing is usually conceived in terms of two cognitive domains with sensory-discriminative and affective-motivational components. The ACC and MCC are thought to mediate the latter of these components. The nociceptive properties of cingulate neurons include large somatic receptive fields and a predominance of nociceptive activations, with some that even respond to an innocuous tap. These responses are predicted by the properties of midline and intralaminar thalamic neurons that project to the cingulate cortex, including the parafascicular, paraventricular and reuniens nuclei that derive their nociceptive information from the spinal cord, the subnucleus reticularis dorsalis and the parabrachial nuclei.
-
Human functional imaging studies of activity during acute noxious stimulation of cutaneous, muscular and visceral tissues show that cutaneous activity is greatest throughout the MCC, whereas the pACC, and to a lesser extent the aMCC, are active during noxious deep tissue stimulation. In addition, electroencephalographic findings indicate that a short-latency nociceptive response can be generated by noxious and innocuous stimuli from the pMCC and dPCC. To investigate the hypothesis that the cingulate cortex mediates pain affect, we localized the simple emotions of sadness, happiness, anger and fear in the framework of cingulate subregions. Amazingly, it failed to show a simple overlap of pain affect sites and sadness as predicted. The five main outcomes of this study are as follows. First, the sACC is involved in negatively valenced memories. Second, the pACC is involved in happy emotions. Third, the aMCC is involved in fear. Fourth, the pMCC and dPCC have little or no involvement in emotion. Fifth, the vPCC is involved in emotion but not in a specific way, as it is also activated by control conditions without emotional valence.
-
Rather than having a simple role in pain affect, the cingulate gyrus seems to have three roles in pain processing. First, the pACC is involved in unpleasant experiences and directly drives autonomic outputs. Second, the aMCC is involved in fear, prediction of negative consequences and avoidance behaviours through the rostral cingulate motor area. Third, the pMCC and dPCC are not involved in emotion but are driven by short-latency somatosensory signals that mediate orientation of the body in space through the caudal cingulate motor area. In addition to these functions, nociceptive stimuli reduce activity in the vPCC and, therefore, activity in a subregion that normally evaluates the self-relevance of incoming visual sensations. So, there is a complex interaction between pain and emotion. Moreover, hypnoanalgesia and opioid and acupuncture placebos indicate mechanisms whereby the cingulate subregions can be engaged for therapeutic intervention.
Abstract
Acute pain and emotion are processed in two forebrain networks, and the cingulate cortex is involved in both. Although Brodmann's cingulate gyrus had two divisions and was not based on any functional criteria, functional imaging studies still use this model. However, recent cytoarchitectural studies of the cingulate gyrus support a four-region model, with subregions, that is based on connections and qualitatively unique functions. Although the activity evoked by pain and emotion has been widely reported, some view them as emergent products of the brain rather than of small aggregates of neurons. Here, we assess pain and emotion in each cingulate subregion, and assess whether pain is co-localized with negative affect. Amazingly, these activation patterns do not simply overlap.
This is a preview of subscription content, access via your institution
Access options
Access to this article via ICE Institution of Civil Engineers is not available.
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aggleton, J. P. The Amygdala (Oxford Univ. Press, New York, USA, 2001).
Davidson, R. J., Scherer, K. R. & Goldsmith, H. H. Handbook of Affective Sciences (Oxford Univ. Press, New York, USA, 2003).
Melzack, R. & Casey, K. L. in The Skin Senses (ed. Kenshalo, D. R.) 423–439 (Thomas, Springfield, Illinois, 1968).
Derbyshire, S. W. G. Exploring the pain neuromatrix. Curr. Rev. Pain 6, 467–477 (2000).
Peyron, R., Laurent, B. & Garcia-Larrea, L. Functional imaging of brain responses to pain: a review and meta-analysis. Neurophysiol. Clin. 30, 263–288 (2000).
Vogt, B. A., Sikes, R. W. & Vogt, L. J. in Neurobiology of Cingulate Cortex and Limbic Thalamus (eds Vogt, B. A. & Gabriel, M.) 19–70, 313–344 (Birkhäuser Boston, Massachusetts, USA, 1993). This review provides the first definition of the medial pain system from nociceptors to the cerebral cortex through the MITN, including circuits, neurophysiology and opioid-receptor binding. Previous efforts emphasized the importance of the thalamus for the two systems because the projections of nociceptive thalamic nuclei were not fully understood. Chapter 1 of this book reports, for the first time, the logical and factual basis of the midcingulate region in monkey and rabbit brains.
MacLean, P. The Triune Brain in Evolution; Role in Paleocerebral Functions (Plenum, New York, USA, 1990).
Bush, G. et al. Dorsal anterior cingulate cortex: a role in reward-based decision-making. Proc. Natl Acad. Sci. USA 99, 523–528 (2002).
Rolls, E. T. et al. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex 13, 308–317 (2003).
Bartels, A. & Zeki, S. The neural basis of romantic love. Neuroreport 11, 3829–3834 (2000).
Craig, A. D. Pain mechanisms: labeled lines versus convergence in central processing. Annu. Rev. Neurosci. 26, 1–30 (2003).
Neafsey, E. J., Terreberry, R. R., Hurley, K. M., Ruit, K. G. & Frysztak, R. J. in Neurobiology of Cingulate Cortex and Limbic Thalamus (eds Vogt, B. A. & Gabriel, M.) 206–223 (Birkhäuser Boston, Massachusetts, USA, 1993). Reviews the evidence for the concept that area 25 is a visceromotor control region, based on electrical stimulation and connection studies, mainly in rodents. Unravelling the mechanism of autonomic regulation by the sACC is pivotal to understanding the subdivisions of the ACC into its subgenual and pregenual parts.
Buchanan, S. L. & Powell, D. A. in Neurobiology of Cingulate Cortex and Limbic Thalamus (eds Vogt, B. A. & Gabriel, M.) 206–223 (Birkhäuser Boston, Massachusetts, USA, 1993).
Sikes, R. W. & Vogt, B. A. Nociceptive neurons in area 24 of rabbit cingulate cortex. J. Neurophsyiol. 68, 1720–1731 (1992).
Vogt, B. A., Vogt, L. J., Nimchinsky, E. A. & Hof, P. R. in Handbook of Chemical Neuroanatomy (eds Bloom, F. E., Björkund, A. & Hökfelt, T.) 455–528 (Elsevier, San Diego, 1997).
Vogt, B. A., Hof, P. R. & Vogt, L. J. in The Human Nervous System 2nd edn (eds Paxinos, G. & Mai, J. K.) 915–949 (Academic, 2004).
Vogt, B. A., Berger, G. R. & Derbyshire, S. W. J. Structural and functional dichotomy of human midcingulate cortex. Eur. J. Neurosci. 18, 3134–3144 (2003).
Vogt, B. A., Vogt, L., Farber, N. B. & Bush, G. Architecture and neurocytology of monkey cingulate gyrus. J. Comp. Neurol. 485, 218–239 (2005).
George, M. S. et al. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 152, 341–351 (1995). One of the first demonstrations of where in the cingulate gyrus memories with negative valences are localized — in the sACC. This subregion provides a substrate for vulnerability to major depression.
Ploner, M., Gross, J., Timmermann, L. & Schnitzler, A. Cortical representation of first and second pain sensation in humans. Proc. Natl Acad. Sci. 99, 12444–12448 (2002).
Dum, R. P. & Strick, P. L. The origin of corticospinal projections from the premotor areas in the frontal lobe. J. Neurosci. 11, 667–689 (1991).
Morecraft, R. J. & Van Hoesen, G. W. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J. Comp. Neurol. 322, 471–489 (1992).
Shima, K. et al. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J. Neurophysiol. 65, 188–202 (1991). This study provides the neurophysiological characterization of the two cingulate motor areas. These motor areas are key outputs from the cingulate gyrus for mediating skeletomotor functions, they are pivotal to the subregional differentiation of the MCC into two parts, and they suggest that there are different mechanisms for somatic pain responses. The functions of the cingulate gyrus cannot be understood outside the context of these two motor areas.
Büchel, C. et al. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J. Neurosci. 22, 970–976 (2002).
Ballantine, H. T., Cassidy, W. L., Flanagan, N. B. & Marino, R. Jr. Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J. Neurosurg. 26, 488–495 (1967).
Gabriel, M. in Neurobiology of Cingulate Cortex and Limbic Thalamus (eds Vogt, B. A. & Gabriel, M.) 478–523 (Birkhäuser Boston, Massachusetts, USA, 1993).
Ploner, M., Freund, H. -J. & Schnitzler, A. Pain affect without pain sensation in a patient with a postcentral lesion. Pain 81, 211–214 (1999).
Vogt, B. A., Rosene, D. L. & Pandya, D. N. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science 204, 205–207 (1979). The first demonstration of MITN projections to the cingulate cortex. These nuclei were later shown to project to many limbic areas in the primate cerebral cortex, including the anterior insula and orbitofrontal cortex, as well as the amygdala, and this projection system might be a network integrator for the limbic/medial parts of the pain neuromatrix.
Casey, K. L. Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J. Neurophysiol. 29, 727–750 (1966).
Dong, W. K., Ryu, H. & Wagman, I. H. Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J. Neurophysiol. 41, 1592–1613 (1978).
Lenz, F. A. et al. Painful stimuli evoke potentials recorded over the human anterior cingulate gyrus. J. Neurophysiol. 79, 2231–2234 (1998).
Kulkarni, B. et al. Attention to pain localization and unpleasantness discriminate the functions of the medial and lateral pain systems. Eur. J. Neurosci. (in the press).
Vogt, B. A., Derbyshire, S. W. J. & Jones, A. K. P. Pain processing in four regions of human cingulate cortex localized with coregistered PET and MR imaging. Eur. J. Neurosci. 8, 1461–1473 (1996).
Derbyshire, S. W. G., Jones, A. K. P. & Gyulai, F. Pain processing during three levels of noxious stimulation produces differential patterns of cerebral activity. Pain 73, 431–445 (1997).
Coghill, R. C., Sang, C. N., Maisog, J. M. & Iadarola, M. J. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J. Neurophysiol. 82, 1934–1943 (1999).
Strigo, I. A., Duncan, G. H., Boivin, M. & Bushnell, M. C. Differentiation of visceral and cutaneous pain in the human brain. J. Neurophysiol. 89, 3294–3303 (2003).
Binkofski, F. et al. Somatic and limbic cortex activation in esophageal distension: a functional magnetic resonance imaging study. Ann. Neurol. 44, 811–815 (1998).
Denton, D. et al. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc. Natl Acad. Sci. USA 96, 2532–2537 (1999).
Matsuura, S. et al. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J. Urol 168, 2035–2039 (2002).
Mertz, H. et al. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distension. Gastroenterology 118, 842–848 (2000).
Naliboff, B. D. et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom. Med. 63, 365–375 (2001).
Zald, D. H., Lee, J. T., Fluegel, K. W. & Pardo, J. V. Aversive gustatory stimulation activates limbic circuits in human. Brain 121, 1143–1154 (1998).
Svensson, P., Minoshima, S., Beydoun, A., Morrow, T. J. & Casey, K. L. Cerebral processing of acute skin and muscle pain in humans. J. Neurophysiol. 78, 450–460 (1997).
Villanueva, L., Cliffer, K. D., Sorkin, L. S., Le Bars, D. & Willis, W. D. Jr. Convergence of heterotopic nociceptive information onto neurons of caudal medullary reticular formation in monkey (Macacca fascicularis). J. Neurophysiol. 63, 1118–1127 (1990). The first demonstration of the functional properties of neurons in the pronociceptive SRD nucleus in the monkey. As this nucleus projects to the parafascicular nucleus in the thalamus, it is a pivotal source of nociceptive input to the cingulate gyrus and partially explains the large receptive fields of nociceptive neurons in the cingulate gyrus.
Villanueva, L., Debois, C., Le Bars, D. & Bernard, J. -F. Organization of diencephalic projections from the medullary subnucleus reticularis dorsalis: a retrograde and anterograde tracer study in the rat. J. Comp. Neurol. 390, 133–160 (1998).
Bester, H., Bourgeais, L., Villanueva, L., Besson, J. -M. & Bernard, J. -F. Differential projections to the intralaminar and gustatory thalamus from the parabrachial area: a PHA-L study in the rat. J. Comp. Neurol. 405, 421–449 (1999).
Saper, C. B. Pain as a visceral sensation. Prog. Brain Res. 122, 237–243 (2000).
Hatanka, N. et al. Thalamocortical and intracortical connections of monkey cingulate motor areas. J. Comp. Neurol. 462, 121–138 (2003).
Schlereth, T., Baumgärtner, U., Magerl, W., Stoeter, P. & Treede, R. -D. Left-hemisphere dominance in early nociceptive processing in the human parasylvian cortex. Neuroimage 20, 441–454 (2003).
Bentley, D. E., Derbyshire, S. W. G., Youell, P. D. & Jones, A. K. P. Caudal cingulate cortex involvement in pain processing: an inter-individual laser evoked potential source localization study using realistic head models. Pain 102, 265–271 (2003).
Niddam, D. M., Chen, L. -F., Yu-Te, W. & Hsieh, J. -C. Spatiotemporal brain dynamics in response to muscle stimulation. Neuroimage 25, 942–951 (2005).
Huang, M. -X., Harrington, D. L., Paulson, K. M., Weisend, M. P. & Lee, R. R. Temporal dynamics of ipsilateral and contralateral motor activity during voluntary finger movement. Hum. Brain Mapp. 23, 26–39 (2004).
Phan, K. L., Wager, T., Taylor, S. F. & Liberzon, I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16, 331–348 (2002).
Vogt, B. A. & Pandya, D. N. Cingulate cortex of rhesus monkey. II. Cortical afferents. J. Comp. Neurol. 262, 271–289 (1987).
Whalen, P. J. et al. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 18, 411–418 (1998).
Bernard, J. F., Huang, G. F. & Besson, J. M. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J. Neurophysiol. 68, 551–569 (1992).
Simpson, J. R., Drevets, W. C., Snyder, A. Z., Gusnard, D. A. & Raichle, M. E. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proc. Natl Acad. Sci. USA 98, 688–693 (2001).
Porro, C. A. et al. Does anticipation of pain affect cortical nociceptive systems? J. Neurosci. 22, 3206–3214 (2002).
Faymonville, M. -E. et al. Neural mechanisms of antinociceptive effects of hypnosis. Anesthesiology 92, 1257–1267 (2000).
Rainville, P., Duncan, G. H. & Price, D. D. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971 (1997).
Vogt, B. A., Watanabe, H., Grootoonk, S. & Jones, A. K. P. Topography of diprenorphine binding in human cingulate gyrus and adjacent cortex derived from PET and MR images. Hum. Brain Mapp. 3, 1–12 (1995).
Petrovic, P., Kalso, E., Petersson, K. M. & Ingvar, M. Placebo and opioid analgesia — imaging a shared neuronal network. Science 295, 1737–1740 (2002). The first study to show colocalization of opioid binding and the opioid placebo in the cingulate cortex.
Zubieta, J. -K. et al. Regulation of human affective responses by anterior cingulate and limbic μ-opioid neurotransmission. Arch. Gen. Psychiatry 60, 1145–1153 (2003).
Pariente, J., White, P., Frackowiak, R. S. J. & Lewith, G. Expectancy and belief modulate the neuronal substrates of pain treated by acupuncture. Neuroimage 25, 1161–1167 (2005).
Adler, L. J. et al. Regional brain activity associated with fentanyl analgesia elucidated by positron emission tomography. Anesth. Analg. 84, 120–126 (1997).
Bantick, S. J. et al. Imaging how attention modulates pain in humans using functional MRI. Brain 125, 310–319 (2002).
Becerra, L. R. et al. Human brain activation under controlled thermal stimulation and habituation to noxious heat: an fMRI study. Magn. Reson. Med. 41, 1044–1057 (1999).
Bornhovd, K. et al. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain 125, 1326–1336 (2002).
Brooks, J. C. W., Nurmikko, T. J., Bimson, W. E., Singh, K. D. & Roberts, N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage 15, 293–301 (2002).
Casey, K. L., Morrow, T. J., Lorenz, J. & Minoshima, S. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J. Neurophysiol. 85, 951–959 (2001).
Casey, K. L., Minoshima, S., Morrow, T. J. & Koeppe, R. A. Comparison of human cerebral activation patterns during cutaneous warmth, heat pain, and deep cold pain. J. Neurophysiol. 76, 571–581 (1996).
Coghill, R. C. et al. Distributed processing of pain and vibration by the human brain. J. Neurosci. 14, 4095–4108 (1994).
Craig, A. D., Reiman, E. M., Evans, A. & Bushnell, M. C. Functional imaging of an illusion of pain. Nature 384, 258–260 (1996).
Davis, K. D., Kwan, C. L., Crawley, A. P. & Mikulis, D. J. Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold and tactile stimuli. J. Neurophysiol. 80, 1533–1546 (1998).
Derbyshire, S. W. G. & Jones, A. K. P. Cerebral responses to a continual tonic pain stimulus measured using positron emission tomography. Pain 76, 127–135 (1998).
Derbyshire, S. W. G. et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage 16, 158–168 (2002).
Derbyshire, S. W. G. et al. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J. Neurol. Neurosurg. Psychiatry 57, 1166–1172 (1994).
Gelnar, P. A., Krauss, B. R., Sheehe, P. R., Szeverenyi, N. M. & Apkarian, A. V. A comparative fMRI study of cortical representations for thermal painful, vibrotactile, and motor performance tasks. Neuroimage 10, 460–482 (1999).
Hofbauer, R. K., Rainville, P., Duncan, G. H. & Bushnell, M. C. Cortical representation of the sensory dimension of pain. J. Neurophysiol. 86, 402–411 (2001).
Jones, A. K. P., Brown, W. D., Friston, K. J., Qi, L. Y. & Frackowiak, R. S. J. Cortical and subcortical localization of response to pain in man using positron emission tomography. Proc. R. Soc. Lond. 244, 39–44 (1991).
Kwan, C. L., Crawley, A. P., Mikulis, D. J. & Davis, K. D. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain 85, 359–374 (2000).
Kurata, J., Thulborn, K. R., Gyulai, F. E. & Firestone, L. L. Early decay of pain-related cerebral activation in functional magnetic resonance imaging. Anesthesiology 96, 35–44 (2002).
Paulson, P. M., Minoshima, S., Morrow, T. J. & Casey, K. L. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain 76, 223–229 (1998).
Peyron, R. et al. Parietal and cingulate processes in central pain. A combined positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) study of an unusual case. Pain 84, 77–87 (2000).
Ploghaus, A. et al. Dissociating pain from its anticipation in the human brain. Science 284, 1979–1981 (1999).
Svensson, P. et al. Cerebral blood-flow changes evoked by two levels of painful heat stimulation: a positron emission tomography study in humans. Eur. J. Pain 2, 95–107 (1998).
Talbot, J. D. et al. Multiple representations of pain in human cerebral cortex. Science 251, 1355–1358 (1991).
Tolle, T. R. et al. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann. Neurol. 45, 40–47 (1999).
Tracey, I. et al. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci. Lett. 288, 159–162 (2000).
Xu, X. et al. Functional localization of pain perception in the human brain studied by PET. Neuroreport 8, 555–559 (1997).
Alexander, G. E. et al. Individual differences in PET activation of object perception and attention systems predict face matching accuracy. Neuroreport 10, 1965–1971 (1999).
Bernstein, L. J., Beig, S., Siegenthaler, A. L. & Grady, C. L. The effect of encoding strategy on the neural correlates of memory for faces. Neuropsychologia 40, 86–89 (2002).
Damasio, A. R. et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neurosci. 3, 1049–1056 (2000).
Dolan, R. J., Morris, J. S. & de Gelder, B. Crossmodal binding of fear in voice and face. Proc. Natl Acad. Sci. USA 98, 10006–10010 (2001).
Dougherty, D. D. et al. Anger in healthy men: a PET study using script-driven imagery. Biol. Psychiatry 46, 466–472 (1999).
Druzgal, T. J. & D'Esposito, M. A neural network reflecting decisions about human faces. Neuron 32, 947–955 (2001).
Fink, G. R. et al. Cerebral representation of one's own past: neural networks involved in autobiographical memory. J. Neurosci. 16, 4275–4282 (1996).
Gemar, M. C., Kapur, S., Segal, Z. V., Brown, G. M. & Houle, S. Effects of self-generated sad mood on regional cerebral activity: a PET study in normal subjects. Depression 4, 81–88 (1996).
George, M. S., Ketter, T. A., Parekh, P. I., Herscovitch, P. & Post, R. M. Gender differences in regional cerebral blood flow during transient self-induced sadness or happiness. Biol. Psychiatry 40, 859–871 (1996).
Izard, C. E. et al. The ontogeny and significance of infants' facial expressions in the first 9 months of life. Dev. Psychol. 31, 997–1013 (1995).
Kesler-West, M. L. et al. Neural substrate of facial emotion processing using fMRI. Brain Res. Cogn. Brain Res. 11, 213–226 (2001).
Liotti, M. et al. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorders. Biol. Psychiatry 48, 30–42 (2000).
Maddock, R. J., Garrett, A. S. & Buonocore, M. H. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience 104, 667–676 (2001).
Maguire, E. A. & Mummery, C. J. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus 9, 54–61 (1999).
Mayberg, H. S. et al. Reciprocal limbic-cortical function and negative mood; converging findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682 (1999).
Morris, J. S. et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature 383, 812–815 (1996).
Morris, J. S. et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain 121, 47–57 (1998).
Phillips, M. L. et al. Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res. 83, 127–138 (1998).
Pietrini, P., Guazzelli, M., Baso, G., Jaffe, K. & Grafman, J. Neural correlates of imaginal aggressive behavior assessed by positron emission tomography in healthy subjects. Am. J. Psychiatry 157, 1772–1781 (2000).
Shah, N. J. et al. The neural correlates of person familiarity: a functional magnetic resonance imaging study with clinical implications. Brain 124, 804–815 (2001).
Sprengelmeyer, R., Rausch, M., Eysel, U. T. & Przuntek, H. Neural structures associated with recognition of facial expressions of basic emotions. Proc. R. Soc. Lond. B 265, 1927–1931 (1998).
Williams, L. M. et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage 14, 1070–1079 (2001).
Acknowledgements
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- LABELLED-LINE THEORIES
-
These predict that a line or projection from lamina I of the spinal cord is specific for nociceptive stimulation and that this line is maintained throughout the CNS; that is, through the thalamus and directly to parts of the cerebral cortex. There is no evidence for a labelled line in the cingulate gyrus.
Rights and permissions
About this article
Cite this article
Vogt, B. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6, 533–544 (2005). https://doi.org/10.1038/nrn1704
Issue Date:
DOI: https://doi.org/10.1038/nrn1704
This article is cited by
-
Decoding auditory deprivation: resting-state fMRI insights into deafness and brain plasticity
Brain Structure and Function (2024)
-
Loosely synchronized activation of anterior cingulate cortical neurons for scratching response during histamine-induced itch
Molecular Brain (2023)
-
Alexithymia characteristics are associated with salience network activity in healthy participants: an arterial spin labeling study
Journal of Physiological Anthropology (2023)
-
Age-related attenuation of cortical synaptic tagging in the ACC is rescued by BDNF or a TrkB receptor agonist in both sex of mice
Molecular Brain (2023)
-
TRPA1 as a O2 sensor detects microenvironmental hypoxia in the mice anterior cingulate cortex
Scientific Reports (2023)